No definitive conclusions on the effects of Gentiopicrin metformin for CRC can be drawn at the molecular level. Up till now, the prognostic significance for diabetic patients with CRC using metformin in survival outcomes has not been systematically assessed. And recent meta-analysis only investigated the general cancer outcomes and all-cause mortality. However, as a systemic disease which can involve different organs to various extent, diabetes can show variation between different cancer types on prognostic effects. Therefore, we performed a systemic review and meta-analysis to evaluate the effect of metformin on survival outcomes in diabetic patients with CRC. As the two most common diseases worldwide, diabetes and colorectal cancer share many risk factors. Previous metaanalyses have demonstrated that type 2 diabetes is associated with increased risk of CRC and metformin is a commonly prescribed anti-diabetic agent in outpatients. We sought to comprehensively investigate the relationship between metformin exposure and CRC outcomes in patients with diabetes by pooling survival data from all studies. In fact, our meta-analysis including 23,255 participants from six cohort studies revealed that diabetic patients with CRC taking metformin achieved an estimated OS benefit of 44% compared with non-metformin users. The potential antitumor effect for metformin has not been fully elucidated, although several observational studies have reported such a trend. Metformin mediates mammalian target of rapamycin pathway via the activation of AMPK and tuberous sclerosis complex 2, phosphorylation of TSC2 which leads to an inhibition of mTOR signaling and reduction in protein synthesis for cancer cells. It also promotes p53-dependent autophagy and cell cycle arrest through a decrease in cyclin D1 protein level. Although experimental data indicate that metformin leads to mTOR inhibition, at present no mTOR inhibitors have been approved for the treatment of CRC patients. Our meta-analysis might be a consistent finding among different population cohorts even though potential confounding factors and risk of publication bias do exist. Though the observational studies cannot be 10-Gingerol interpretated as causation, several aspects can be taken into consideration recommended by Hill et al as evidence to support our conclusion. First, temporal association between metformin exposure and CRC incidence, which means CRC occurs after metformin exposure in all studies. Second, the results reveal a biological gradient or dose-response relationship in one study, with higher intensity metformin users exclusively having lower risk of CRC-specific mortality. Third, what we find is also biologically plausible as causality with the fact that metformin is found to reduce cancer cell proliferation, inhibit mTOR and protein synthesis and lead to cell cycle arrest. Fourth, consistent results are shown 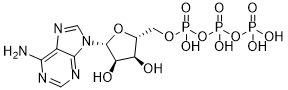 in the forest plot for both individual study and pooled estimates of the six included studies. Fifth, significant strength of the association between metformin users and non users is revealed for an estimated OS benefit of over 40%. Several potential limitations to our meta-analysis need to be addressed. First, we did not fully investigate the heterogeneity of individual studies.
in the forest plot for both individual study and pooled estimates of the six included studies. Fifth, significant strength of the association between metformin users and non users is revealed for an estimated OS benefit of over 40%. Several potential limitations to our meta-analysis need to be addressed. First, we did not fully investigate the heterogeneity of individual studies.