Significantly, activated microglia have also been found in the subretinal space of patients with AMD, and are juxtaposed in close proximity with RPE cells overlying drusen. Additionally, in a number of mouse models for AMD, involving the absence of chemokine ligands/receptors, CCL2 and/or CX3CR1, microglia accumulation in the subretinal space was accelerated and more pronounced. This accumulation of subretinal microglia was in turn associated with multiple features reminiscent of AMD histopathology including the accumulation of drusen-like deposits, local RPE structural changes, and CNV formation. The anatomical separation between retinal microglia and RPE cells under normal conditions and their juxtaposition into direct and intimate contact in the subretinal space under pathological situations suggest that cellular interactions between these two retinal cell types may be of particular pathogenetic significance. Microglia, serving as resident immune cells of the retina, can have multiple functional states and carry out diverse functions. Capable of rapid dynamism and motility, they also synthesize and release multiple cytokines, chemokines, neurotrophic factors, and neurotransmitters that allow them to interact with multiple CNS cell types and exert cytotoxic or cytoprotective effects depending on the tissue context. While there has been evidence that retinal microglia may interact with signals from retinal neurons, photoreceptors, and retinal vessels, the direct influences of retinal microglia on RPE structure, physiology, and gene expression have not been previously addressed. An understanding of the nature of this cell-cell interaction has the potential to shed useful light on the cellular basis of the inflammatory etiology of AMD. In this study, we examined the nature of microglia-induced effects on RPE cells using an 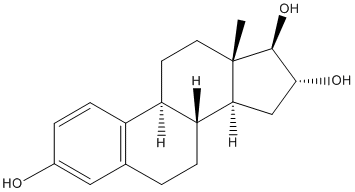 in vitro co-culture model where cultured activated mouse retinal microglia were co-cultured with primary RPE cells from animals of the same genetic background. We examined the consequences of Ginsenoside-F2 microglial co-culture on RPE structure, physiology, and the expression and secretion of molecules important in influencing the inflammatory and angiogenic environment in the subretinal space. In addition, we employed an in vivo model of microglia-RPE interactions by transplanting cultured microglia into the subretinal space of experimental mice. Our results show that signals from activated retinal microglia prominently alter the structure, properties, and gene expression of RPE cells both in vitro and in vivo. These alterations constitute a disorganization of the RPE monolayer and create a pro-inflammatory environment in the subretinal space conducive for the further recruitment of retinal microglia and for the formation of CNV. The similarities between these alterations and the important clinical features found in AMD strongly suggest that microglia-RPE interactions play an important and determining role in the pathogenesis of AMD. Microglia-RPE interactions may represent a potential locus of therapeutic intervention in the treatment and prevention of Ginsenoside-F4 vision loss in AMD. In the present study, we found that as a consequence of coming into close proximity with activated retinal microglia, primary RPE cells developed multiple structural and functional alterations. These involved decreased expression levels of visual cycle protein, RPE65, and proteins in the tight junctions, a disruption of ZO-1 localization in cell junctions, and a loss of regular RPE cell shape and distribution.
in vitro co-culture model where cultured activated mouse retinal microglia were co-cultured with primary RPE cells from animals of the same genetic background. We examined the consequences of Ginsenoside-F2 microglial co-culture on RPE structure, physiology, and the expression and secretion of molecules important in influencing the inflammatory and angiogenic environment in the subretinal space. In addition, we employed an in vivo model of microglia-RPE interactions by transplanting cultured microglia into the subretinal space of experimental mice. Our results show that signals from activated retinal microglia prominently alter the structure, properties, and gene expression of RPE cells both in vitro and in vivo. These alterations constitute a disorganization of the RPE monolayer and create a pro-inflammatory environment in the subretinal space conducive for the further recruitment of retinal microglia and for the formation of CNV. The similarities between these alterations and the important clinical features found in AMD strongly suggest that microglia-RPE interactions play an important and determining role in the pathogenesis of AMD. Microglia-RPE interactions may represent a potential locus of therapeutic intervention in the treatment and prevention of Ginsenoside-F4 vision loss in AMD. In the present study, we found that as a consequence of coming into close proximity with activated retinal microglia, primary RPE cells developed multiple structural and functional alterations. These involved decreased expression levels of visual cycle protein, RPE65, and proteins in the tight junctions, a disruption of ZO-1 localization in cell junctions, and a loss of regular RPE cell shape and distribution.