In the METH-treated flies, suggesting that METH either induces an anaerobic response or a Warburglike effect or some third hitherto unknown process. The heat shock protein 60, primarily a mitochondrial Hsp, is also an indicator of the aerobic status of the cell and is involved in the apoptotic response. Hsp60 is expressed at high levels in normal cells, but hypoxia 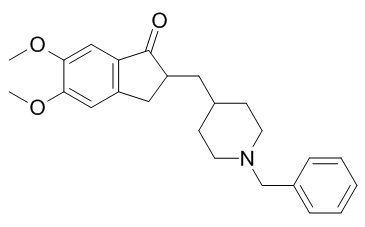 decreases its expression and/or changes its cellular distribution, causing apoptosis. Indeed, decreasing the level of Hsp60 in cardiac myocytes was sufficient to cause apoptosis. Hsp60 forms a macromolecular complex with the pro-apoptotic protein BAX, which blocks the ability of BAX to translocate to the mitochondria and to promote apoptosis in vivo. The Folinic acid calcium salt pentahydrate reduced expression of the Hsp60 protein in METH-treated flies, which we observed, supports the idea that METH induces hypoxia. Levels of the mitochondrial ATP synthase, bellwether, increased with METH exposure. The over-expression of the subunits of the catalytic core of the mitochondrial F �CF ATP synthase complex, including the alpha-subunit, are correlated with apoptosis. Up-regulation of these proteins apparently causes a transient increase in intracellular ATP levels, which is necessary for apoptosis; pharmacologically inhibiting ATP synthase blocks apoptosis. Apoptosis is induced in response to a specific signal that indicates an imbalance between aerobic and anaerobic ATP biosynthesis. Several oncogenes have been implicated in the Warburg effect, including the serine-threonine kinases that enhance glucose uptake and aerobic glycolysis in cancer cells and are able to do so independently of hypoxia-inducible factor; the levels of two AKT proteins significantly changed in the METH-treated flies. AKT mobilizes glucose transporters to the cell surface to enhance glucose uptake and activates hexokinase, a protein that was 3,4,5-Trimethoxyphenylacetic acid over-expressed in the METH-treated flies. Elstrom and co-workers reported that through these effects, AKT is able to enhance glycolytic flux without affecting mitochondrial oxidative phosphorylation, thereby presumably contributing to the Warburg effect. Moreover, the AKT and phosphatidylinositol triphosphate kinase protein levels were up-regulated in METH-treated flies. The PI3K-AKT signaling pathway promotes cell growth, increases glucose uptake, influences cell cycle progression, and prevents apoptosis through multiple mechanisms. The transcription factor c-Myc, a known oncogene, regulates the cell cycle, differentiation, apoptosis, metabolism, and cellular responses to oxidative stress. Typically, the expression of c-Myc is tightly regulated by multiple transcriptional activators and repressors. In METH-treated Drosophila, however, multiple genes that regulate c-Myc were differentially expressed. For instance, YY1 transcription factor, which has previously been associated molecular responses to oxidative stress and heart disease, activates the transcription of Notch 1 transcription factor. Subsequently, the N1IC-YY1 complex binds to the major promoter of the c-Myc gene and activates its expression. In addition, enolase, which was up-regulated in METH-treated flies, and promoter binding protein 1, which results from an alternative translation initiation codon of the enolase gene, are transcriptional repressors of c-Myc. The simultaneous upregulation of transcriptional activators and repressors suggests that METH disrupts the fine control of c-Myc. Interestingly, c-Myc has been associated with the direct activation of aerobic glycolysis in human cancers. Numerous METH-responsive glycolytic genes and proteins detected in our microarray and proteomic analysis are known to interact with cMyc. For example, c-Myc activates many glycolytic genes, including hexokinase and enolase, both of which were over-expressed in METH-treated flies. Increased glycolytic activity requires increased glucose uptake via glucose transporter proteins and the increased expression of glycolytic enzymes. METH treatment induced changes in the flies’ expression of glucose transporters, adolase, and glyceraldehyde-3-phosphate dehydrogenase.
decreases its expression and/or changes its cellular distribution, causing apoptosis. Indeed, decreasing the level of Hsp60 in cardiac myocytes was sufficient to cause apoptosis. Hsp60 forms a macromolecular complex with the pro-apoptotic protein BAX, which blocks the ability of BAX to translocate to the mitochondria and to promote apoptosis in vivo. The Folinic acid calcium salt pentahydrate reduced expression of the Hsp60 protein in METH-treated flies, which we observed, supports the idea that METH induces hypoxia. Levels of the mitochondrial ATP synthase, bellwether, increased with METH exposure. The over-expression of the subunits of the catalytic core of the mitochondrial F �CF ATP synthase complex, including the alpha-subunit, are correlated with apoptosis. Up-regulation of these proteins apparently causes a transient increase in intracellular ATP levels, which is necessary for apoptosis; pharmacologically inhibiting ATP synthase blocks apoptosis. Apoptosis is induced in response to a specific signal that indicates an imbalance between aerobic and anaerobic ATP biosynthesis. Several oncogenes have been implicated in the Warburg effect, including the serine-threonine kinases that enhance glucose uptake and aerobic glycolysis in cancer cells and are able to do so independently of hypoxia-inducible factor; the levels of two AKT proteins significantly changed in the METH-treated flies. AKT mobilizes glucose transporters to the cell surface to enhance glucose uptake and activates hexokinase, a protein that was 3,4,5-Trimethoxyphenylacetic acid over-expressed in the METH-treated flies. Elstrom and co-workers reported that through these effects, AKT is able to enhance glycolytic flux without affecting mitochondrial oxidative phosphorylation, thereby presumably contributing to the Warburg effect. Moreover, the AKT and phosphatidylinositol triphosphate kinase protein levels were up-regulated in METH-treated flies. The PI3K-AKT signaling pathway promotes cell growth, increases glucose uptake, influences cell cycle progression, and prevents apoptosis through multiple mechanisms. The transcription factor c-Myc, a known oncogene, regulates the cell cycle, differentiation, apoptosis, metabolism, and cellular responses to oxidative stress. Typically, the expression of c-Myc is tightly regulated by multiple transcriptional activators and repressors. In METH-treated Drosophila, however, multiple genes that regulate c-Myc were differentially expressed. For instance, YY1 transcription factor, which has previously been associated molecular responses to oxidative stress and heart disease, activates the transcription of Notch 1 transcription factor. Subsequently, the N1IC-YY1 complex binds to the major promoter of the c-Myc gene and activates its expression. In addition, enolase, which was up-regulated in METH-treated flies, and promoter binding protein 1, which results from an alternative translation initiation codon of the enolase gene, are transcriptional repressors of c-Myc. The simultaneous upregulation of transcriptional activators and repressors suggests that METH disrupts the fine control of c-Myc. Interestingly, c-Myc has been associated with the direct activation of aerobic glycolysis in human cancers. Numerous METH-responsive glycolytic genes and proteins detected in our microarray and proteomic analysis are known to interact with cMyc. For example, c-Myc activates many glycolytic genes, including hexokinase and enolase, both of which were over-expressed in METH-treated flies. Increased glycolytic activity requires increased glucose uptake via glucose transporter proteins and the increased expression of glycolytic enzymes. METH treatment induced changes in the flies’ expression of glucose transporters, adolase, and glyceraldehyde-3-phosphate dehydrogenase.