On the plasma membrane, a2b1 integrin colocalizes first with GPI-anchored proteins but is sorted out from GPIanchored proteins during internalization. However, later, the multivesicular bodies become increasingly positive for caveolin-1 suggesting that stable lipid microdomains exist in these endosomes. Interestingly, our previous intraendosomal pH measurements have shown that a2-MVBs are not acidic and do not associate with lysosomal structures. As these structures have proven to be important for viral uncoating and genome egress for replication, as well as for location of enhanced integrin turnover, the characterization and role 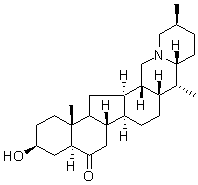 of the lipid microdomains is important to understand the structure and function of these novel non-acidic multivesicular bodies. Many Cinoxacin viruses, such as HIV, hepatitis C virus, West Nile virus, vaccinia virus and poliovirus depend on plasma membrane Gomisin-D cholesterol for efficient cell entry and replication. However, the putative role of these lipids in internalized endosomes has not been demonstrated. The cellular cholesterol concentration varies largely depending on the compartment membrane. Most of the cholesterol can be found at the plasma membrane, but also many intracellular organelles contain raft-like microdomains. In addition, studies on acidic late endosomes have revealed that raft-like membranes rich in cholesterol, sphingomyelin and raft proteins – can be found not only on the limiting membranes but also on the internal membranes of these multivesicular organelles. In late endosomes, cholesterol accumulation can lead to disturbed vesicle trafficking from the structures, underlining the importance of membrane cholesterol in these intracellular organelles. In this study, we investigated whether cholesterol plays a role in ligand uptake, virus uncoating in endosomes and the biogenesis of the non-acidic multivesicular bodies. We showed that the formation of a2-MVBs is highly dependent on cholesterol and that perturbation of cholesterol inhibits collagen and EV1 entry as well as virus uncoating and infection. Cholesterol depletion also decreased strong integrin and aerolysin labeling in the cell boundary and both labels were more scattered on the plasma membrane. Secondly, we labeled plasma membrane cholesterol by filipin staining. This labeling showed also less colocalization after ketoconazole treatment. In addition, a further treatment with cold Triton X-100 caused depletion of integrin labeling after ketoconazole treatment suggesting that integrin had moved out from the detergent-resistant domain to Triton X-100 soluble domain on the plasma membrane. a2 integrin distribution after ketoconazole treatment was further studied in detail with electron microscopy. The micrographs revealed that lipoprotein starved cells showed structures that were already partially multivesicular after 30 min internalization as we had observed before for normal biogenesis of a2-MVBs whereas in ketoconazole-treated cells integrin was found mainly on plasma membrane. After 3.5 h, lipoprotein-starved cells contained a2-MVBs with increasing amounts of intraluminal vesicles and internal membranes compared to 30 min time point as has been shown for a2 integrin internalization pathway previously. In contrast, no integrin containing multivesicular structures were observed in ketoconazole-treated cells, and the few found cytoplasmic structures showed tubular elements that are characteristic of the earliest forms of endosomes after integrin entry, suggesting that the biogenesis of multivesicular structures was halted.
of the lipid microdomains is important to understand the structure and function of these novel non-acidic multivesicular bodies. Many Cinoxacin viruses, such as HIV, hepatitis C virus, West Nile virus, vaccinia virus and poliovirus depend on plasma membrane Gomisin-D cholesterol for efficient cell entry and replication. However, the putative role of these lipids in internalized endosomes has not been demonstrated. The cellular cholesterol concentration varies largely depending on the compartment membrane. Most of the cholesterol can be found at the plasma membrane, but also many intracellular organelles contain raft-like microdomains. In addition, studies on acidic late endosomes have revealed that raft-like membranes rich in cholesterol, sphingomyelin and raft proteins – can be found not only on the limiting membranes but also on the internal membranes of these multivesicular organelles. In late endosomes, cholesterol accumulation can lead to disturbed vesicle trafficking from the structures, underlining the importance of membrane cholesterol in these intracellular organelles. In this study, we investigated whether cholesterol plays a role in ligand uptake, virus uncoating in endosomes and the biogenesis of the non-acidic multivesicular bodies. We showed that the formation of a2-MVBs is highly dependent on cholesterol and that perturbation of cholesterol inhibits collagen and EV1 entry as well as virus uncoating and infection. Cholesterol depletion also decreased strong integrin and aerolysin labeling in the cell boundary and both labels were more scattered on the plasma membrane. Secondly, we labeled plasma membrane cholesterol by filipin staining. This labeling showed also less colocalization after ketoconazole treatment. In addition, a further treatment with cold Triton X-100 caused depletion of integrin labeling after ketoconazole treatment suggesting that integrin had moved out from the detergent-resistant domain to Triton X-100 soluble domain on the plasma membrane. a2 integrin distribution after ketoconazole treatment was further studied in detail with electron microscopy. The micrographs revealed that lipoprotein starved cells showed structures that were already partially multivesicular after 30 min internalization as we had observed before for normal biogenesis of a2-MVBs whereas in ketoconazole-treated cells integrin was found mainly on plasma membrane. After 3.5 h, lipoprotein-starved cells contained a2-MVBs with increasing amounts of intraluminal vesicles and internal membranes compared to 30 min time point as has been shown for a2 integrin internalization pathway previously. In contrast, no integrin containing multivesicular structures were observed in ketoconazole-treated cells, and the few found cytoplasmic structures showed tubular elements that are characteristic of the earliest forms of endosomes after integrin entry, suggesting that the biogenesis of multivesicular structures was halted.