Therefore presumably is subject to regulation by DUBs. Altering ubiquitination pathways may represent a way to modulate antibacterial autophagy and intracellular proliferation of pathogens. The ubiquitin system and the DUB enzymes themselves have become a new class of interesting therapeutic targets. Although no DUB inhibitors are yet in clinical trials, diverse inhibitors have already been described, including the USP14 inhibitor IU1 and inhibitors specific to USP7, USP2 and UCH-L3. In EX 527 addition, a small cell-permeable molecule, WP1130, also known as Desgrasyn, which selectively inhibits a subset of cellular DUBs, has been described recently as a potential anti-cancer therapeutic. This molecule causes depletion of monomeric ubiquitin molecules and accumulation of ubiquitinated proteins in cells. A previous study demonstrated that WP1130 directly inhibits in vitro activity of specific DUBs like USP9x, USP5, USP14 and UCH37, without affecting others, showing some degree of specificity. However, the fullspectrum of WP1130 DUB targets as well as its mechanism of action are still unknown. We previously found that WP1130 has anti-infective activity, reducing intracellular replication of some bacterial and viral pathogens. Replication of murine norovirus in a murine macrophage-like cell line, and other RNA viruses, were significantly reduced by WP1130. This antiviral activity was at least in part mediated by inhibition of USP14, a proteasome associated DUB that also controls induction of the unfolded protein response. Moreover, we recently showed that DUB inhibition by WP1130 increases ASP1517 HIF inhibitor killing of the gram-positive bacterium, Listeria monocytogenes, within macrophages. WP1130 treatment rapidly enhanced localization of the antimicrobial effector iNOS to the bacterial phagosome. These recent observations suggest that perturbation of protein ubiquination in host cells by small molecule DUB inhibitors may be an effective strategy to reduce infection that will cause minimal selective pressure on the pathogen itself. L. monocytogenes and noroviruses, along with Salmonella and Toxoplasma, are among the leading causes of food and waterborne diarrheal diseases worldwide. In the US alone, food-borne pathogens are estimated to cause 9.4 million infections and around 1,350 deaths annually, and 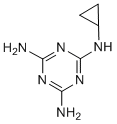 are associated with substantial healthcare costs. However, there are limited vaccines or antimicrobial drugs available to prevent or treat these infections. As the DUB inhibitor WP1130 showed activity against two of these food-borne pathogens, exploiting the ubiquitin system for the development of a new class of broad-spectrum therapeutics to treat these infections is appealing. However, therapeutic use of WP1130 itself is limited due to its low solubility and poor bioavailability in animals. Here we use L. monocytogenes as a model pathogen to screen a small library of WP1130-derivative molecules for antiinfective efficacy in macrophages, a major reservoir for many intracellular pathogens, with the overall goal of finding derivatives with better solubility and anti-infective activity. Several authors recently reported that the incretin system induces an inflammatory and pro-lipolytic response via the PKA NF-kB – IL-1 pathway and impairs insulin sensitivity and glucose uptake in human adipocytes. One of the key mechanisms in the pathogenesis of diabetes-related vascular dysfunction is oxidative stress. Oxidative stress is attributable to excessive production of reactive oxygen species and inflammatory markers by tumor necrosis factor-alpha, macrophage chemotactic protein-1 and other markers. The inflammatory response was reported to downregulate eNOS expression and upregulate iNOS expression in rodents and increase NADH oxidase activity and vascular remodeling.
are associated with substantial healthcare costs. However, there are limited vaccines or antimicrobial drugs available to prevent or treat these infections. As the DUB inhibitor WP1130 showed activity against two of these food-borne pathogens, exploiting the ubiquitin system for the development of a new class of broad-spectrum therapeutics to treat these infections is appealing. However, therapeutic use of WP1130 itself is limited due to its low solubility and poor bioavailability in animals. Here we use L. monocytogenes as a model pathogen to screen a small library of WP1130-derivative molecules for antiinfective efficacy in macrophages, a major reservoir for many intracellular pathogens, with the overall goal of finding derivatives with better solubility and anti-infective activity. Several authors recently reported that the incretin system induces an inflammatory and pro-lipolytic response via the PKA NF-kB – IL-1 pathway and impairs insulin sensitivity and glucose uptake in human adipocytes. One of the key mechanisms in the pathogenesis of diabetes-related vascular dysfunction is oxidative stress. Oxidative stress is attributable to excessive production of reactive oxygen species and inflammatory markers by tumor necrosis factor-alpha, macrophage chemotactic protein-1 and other markers. The inflammatory response was reported to downregulate eNOS expression and upregulate iNOS expression in rodents and increase NADH oxidase activity and vascular remodeling.