At the correlation analysis highlighted that positions are strongly correlated in LPurLs. For sequences where 1181 is a leucine residue, position 1286 AZ 960 905586-69-8 located opposite it, on b42 strand is almost always a leucine or an isoleucine residue. However, in sequence where position 1181 is occupied by a phenylalanine residue, position 1286 is almost always a methionine residue. Besides, it has been previously shown breaking amino acid correlation networks can yield deleterious effects on certain consensus mutations. Moreover, these co-evolving residues could be a part of a large network, thus posing challenges in designing compensatory mutations due to cascading effects. Since SCA on PurLs indicated that L1181 is part of such an evolutionarily link, it is no longer surprising that stand alone mutations at this site perturb the connectivity, thereby resulting in destabilization. On the other hand, evolutionary statistics reveal that presence of tryptophan residue at position 1181 does not break the correlation analysis even though it occurs with low frequency at this position. Although the X-ray structure for this mutant is not yet available, based on the crystal structure of F209W it can be concluded that L1181W mutation is tolerated by adjustment in structure of the nearby surface exposed loop, via perturbations in the surrounding region without affecting structure, stability and function. In order to propose a model for allosteric regulation via Xe1 cavity, a structural superposition performed on various ammonia producing enzymes CPS, IGP synthase and PurF. The structural analyses revealed that this cavity was unique to StPurL, while in other structures this space where xenon was found is filled by none other than a phenylalanine residue. Closer examination revealed that the C-terminal helix of StPurL adjacent to the Xe1 site was pushed back, and as a consequence did not superimpose well with the helices in the other glutaminase structures thereby forming the cavity where xenon was bound. A similar scenario was also observed in thermotoga maritima PurQ where this corresponding helix also superimposed 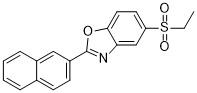 well with StPurL and did not align with the rest of the glutaminases. In addition, it was noted that the loop containing the residues of the oxyanion hole that are important for subsequent hydrolysis of the thioester intermediate, while having a good overlap between CPS, IGP synthase and GMP synthase, had a bad overlap with StPurL and TmPurQ. Structures of the StPurL protein and the TmPurQ proteins have been reported in an inactive form, where the thioester intermediate is covalently attached to the active site cysteine residue. However, the other representative structures are either in the apo form or with non-covalent inhibitors bound. Therefore, differences in conformation of the helix among these structures may shed light on some of the movements that take place during allosteric regulation of the protein. Since crystal structure is only a static representation it is possible that the cavity observed in StPurL may provide “breathing space” for these movements to occur and consequently these spaces may be occupied during the course of the reaction. Based on the above evidence we propose that upon activation, a Kinase Inhibitor Library customer reviews forward motion of helix a36 occurs which, perturbs the beta strand, b42, forming the opposite wall of the xenon binding cavity. This strand is directly connected to the oxyanion hole residues and a perturbation in this region initiates the correct positioning of the active site via restructuring of the oxyanion hole. Hence, we suggest that this cavity is transient and in the alternate conformations of the protein this cavity may be occupied and this space cannot be arbitrarily filled.
well with StPurL and did not align with the rest of the glutaminases. In addition, it was noted that the loop containing the residues of the oxyanion hole that are important for subsequent hydrolysis of the thioester intermediate, while having a good overlap between CPS, IGP synthase and GMP synthase, had a bad overlap with StPurL and TmPurQ. Structures of the StPurL protein and the TmPurQ proteins have been reported in an inactive form, where the thioester intermediate is covalently attached to the active site cysteine residue. However, the other representative structures are either in the apo form or with non-covalent inhibitors bound. Therefore, differences in conformation of the helix among these structures may shed light on some of the movements that take place during allosteric regulation of the protein. Since crystal structure is only a static representation it is possible that the cavity observed in StPurL may provide “breathing space” for these movements to occur and consequently these spaces may be occupied during the course of the reaction. Based on the above evidence we propose that upon activation, a Kinase Inhibitor Library customer reviews forward motion of helix a36 occurs which, perturbs the beta strand, b42, forming the opposite wall of the xenon binding cavity. This strand is directly connected to the oxyanion hole residues and a perturbation in this region initiates the correct positioning of the active site via restructuring of the oxyanion hole. Hence, we suggest that this cavity is transient and in the alternate conformations of the protein this cavity may be occupied and this space cannot be arbitrarily filled.
Month: July 2019
Elucidate the molecular mechanisms and biological pathways implicated in RUNX2-mediated action in EOC cells
We used a complementary gene expression profiling using the DNA microarray technology to monitor cellular changes in gene expression and discover the molecular targets upon RUNX2 suppression in EOC cells. To our knowledge, the present work represents the first effort to define global changes in gene expression upon modulation of RUNX2 expression in cancer cells. We analyzed both functionally related genes that were commonly differentially expressed in SKOV3 EOC cells upon RUNX2 knockdown. The gene expression data and consecutive network and pathway analyses were quite confirmatory of the data obtained by the RUNX2 functional assays. Indeed, microarray data sustained RUNX1 correlation with EOC cell proliferation, migration and invasion, since RUNX2 knockdown resulted in reduced expression of genes associated with metabolism, cellular growth & proliferation and cellular movement, while a number of genes linked to cell death were induced. IPA network analysis was indicative for some important gene nodes linked to RUNX2 suppression in EOC cells, as most of these substantiate and/or complement the functional data obtained. Thus, RUNX2 knockdown resulted in (+)-JQ1 upregulation of gene nodes/genes known to be implicated in apoptosis induction or displaying TSG functions. Notably the UBC interaction network and its members were shown to decrease in anchorage-independent cell growth and increase apoptosis, suggesting UBC may act as a negative regulator of skin carcinogenesis; CRYAB has been reported as a potential TSG, while increased expression of BCL-XS protein in tumors was associated with decreased proliferation and induction of apoptosis. Similarly, CTGF upregulation was found to be associated with apoptosis and decrease of tumor cell invasion; PPM1A expression could induce cell cycle arrest and apoptosis via activation of the p53 pathway, and NF2 has been characterized as a TSG in different cancers. Given the similar roles of RUNX1 and RUNX2 in EOC progression and the fact that all three RUNX proteins recognize common DNA sequence motifs, we analyzed the extent of overlap in differentially expressed genes/functional pathways following RUNX1 and RUNX2 knockdown in the SKOV3 ovarian adenocarcinoma cell line. Both the Venn diagram comparisons, as well as gene clustering and IPA functional analyses were indicative for distinct molecular mechanisms and functional pathways associated with RUNX1 or RUNX2  implication in EOC progression, although both genes could potentially modulate the expression of some common genes involved in EOC disease advancement and metastasis. In conclusion, we have shown that the RUNX2 transcription factor is significantly overexpressed in serous EOC tumors, including LMP tumors, compared to normal ovarian tissue. BSP validation of the RUNX2 methylation status in primary EOC tumors and omental metastasis were indicative for no implication of LY2835219 epigenetics mechanisms in RUNX2 overexpression in metastatic tissues. Further functional analyses of RUNX2 in EOC cells pointed towards its association with EOC cell proliferation, migration and invasion. Gene expression profiling and consecutive network and pathway analyses confirmed these findings, as various genes and pathways known previously to be implicated in ovarian tumorigenesis, including EOC tumor invasion and metastasis, were found to be suppressed upon RUNX2 knockdown, while a number of pro-apoptotic genes and some EOC TSGs were found to be induced. Our data suggest that RUNX2 is possibly implicated in EOC tumor and cancer cell growth and invasion and could represent a potential EOC therapeutic target. The present study also reveals that RUNX1 and RUNX2 employ distinct molecular mechanisms in EOC tumorigenesis despite evident similarities of their action on EOC cell phenotype and behavior.
implication in EOC progression, although both genes could potentially modulate the expression of some common genes involved in EOC disease advancement and metastasis. In conclusion, we have shown that the RUNX2 transcription factor is significantly overexpressed in serous EOC tumors, including LMP tumors, compared to normal ovarian tissue. BSP validation of the RUNX2 methylation status in primary EOC tumors and omental metastasis were indicative for no implication of LY2835219 epigenetics mechanisms in RUNX2 overexpression in metastatic tissues. Further functional analyses of RUNX2 in EOC cells pointed towards its association with EOC cell proliferation, migration and invasion. Gene expression profiling and consecutive network and pathway analyses confirmed these findings, as various genes and pathways known previously to be implicated in ovarian tumorigenesis, including EOC tumor invasion and metastasis, were found to be suppressed upon RUNX2 knockdown, while a number of pro-apoptotic genes and some EOC TSGs were found to be induced. Our data suggest that RUNX2 is possibly implicated in EOC tumor and cancer cell growth and invasion and could represent a potential EOC therapeutic target. The present study also reveals that RUNX1 and RUNX2 employ distinct molecular mechanisms in EOC tumorigenesis despite evident similarities of their action on EOC cell phenotype and behavior.
We have replicated in our laboratory in contrast to MSNs mGluR-dependent LTD is expressed
In summary, our data suggest that dopaminergic function is differentially altered in mesolimbic and nigrostriatal MK-0683 pathways in the Fmr1-/Y mouse model of FXS, the AG-013736 319460-85-0 balance of effects facilitating reward function and diminishing motor function. While the importance of cholinergic actions in the striatum is appreciated, and anticholinergics have been in routine clinical use for extrapyramidal movement disorders for decades, the function of large, aspiny, tonically active cholinergic interneurons remains much less understood than other striatal cell types, such as MSNs and GABAergic fast-spiking interneurons, particularly in the NAc. TANs synapse extensively with MSNs and are the primary source of acetylcholine in both the dorsal striatum and NAc. As is the case for their role in motor function, the euphoric and rewarding effects of muscarinic anticholinergics have been appreciated for centuries, but the exact mechanisms by which anticholinergics function in mesolimbic brain reward circuitry are not well understood. Recent studies have suggested that TANs may integrate thalamostriatal and corticostriatal input and local FSI function to regulate firing of MSNs. Most of what is known regarding TAN function has been learned from experiments in the dorsal striatum, and with a few exceptions these neurons have been largely uninvestigated in the NAc. The net behavioral effects of the M1 antagonist, trihexyphenidyl, in WT mice were potentiation of BSR within a narrow dose range, suppression of maximum operant response rate without a change in threshold at the highest dose tested, and locomotor stimulation at a dose that potentiated BSR. In contrast, trihexyphenidyl did not potentiate BSR in Fmr1-/Y mice at any dose, and the locomotor stimulation we observed at 10 mg/kg was significantly enhanced in Fmr1-/Y compared to WT mice, consistent with a parkinsonian motor phenotype and further illustrating the dissociation of reward and motor effects in Fmr1-/Y mice. Trihexyphenidyl has no effect by itself on dopamine release in the NAc, but increases cocaine-potentiated NAc dopamine release and cocaine-stimulated locomotor activation, suggesting that cholinergic regulation of mesolimbic circuitry normally keeping NAc dopamine release in check is disinhibited 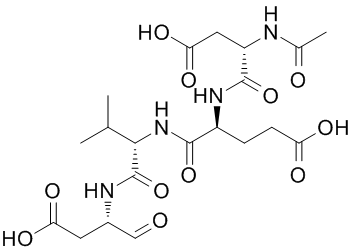 by M1 antagonism, and that this function may be impaired in Fmr1-/Y mice. Acetylcholine signaling through M2/M4 receptors on TANs indirectly reduces dopamine release, but signaling through M1 receptors on presynaptic terminals increases excitability of dopaminergic neurons by inhibiting local GABA release in the VTA. Neither mechanism fully explains our present behavioral results with trihexyphenidyl. We are currently investigating how M1 activity in NAc MSNs differs between Fmr1-/Y and WT mice. Given that M1 receptors are also Gq-coupled and signal through PLC, it is possible that M1 function is also altered in NAc MSNs of mice lacking FMRP. Group I mGluR receptors are distributed ubiquitously throughout the brain and act as a brake on glutamatergic excitation. The highly selective mGluR5 antagonist, MPEP, has anxiolytic potency in rodent models and potentiated BSR in our hands, with larger effects in Fmr1-/Y than in WT mice. MPEP also transiently but significantly stimulated locomotor activity, although relative motor stimulation compared to baseline activity was similar between genotypes. In the dorsal striatum, mGluR5 receptors mediate both postsynaptic AMPA receptor endocytosis, which is enhanced in hippocampal neurons of Fmr1-/Y mice, and presynaptic endocannabinoid -dependent LTD. It has been shown that mGluR5-coupled retrograde eCB signaling through Gq-coupled activation of PLC and diacylglycerol lipase is disrupted in both dorsal striatum and NAc, and, in contrast to hippocampus, mGluR5-dependent LTD is absent from NAc MSNs in Fmr1-/Y mice.
by M1 antagonism, and that this function may be impaired in Fmr1-/Y mice. Acetylcholine signaling through M2/M4 receptors on TANs indirectly reduces dopamine release, but signaling through M1 receptors on presynaptic terminals increases excitability of dopaminergic neurons by inhibiting local GABA release in the VTA. Neither mechanism fully explains our present behavioral results with trihexyphenidyl. We are currently investigating how M1 activity in NAc MSNs differs between Fmr1-/Y and WT mice. Given that M1 receptors are also Gq-coupled and signal through PLC, it is possible that M1 function is also altered in NAc MSNs of mice lacking FMRP. Group I mGluR receptors are distributed ubiquitously throughout the brain and act as a brake on glutamatergic excitation. The highly selective mGluR5 antagonist, MPEP, has anxiolytic potency in rodent models and potentiated BSR in our hands, with larger effects in Fmr1-/Y than in WT mice. MPEP also transiently but significantly stimulated locomotor activity, although relative motor stimulation compared to baseline activity was similar between genotypes. In the dorsal striatum, mGluR5 receptors mediate both postsynaptic AMPA receptor endocytosis, which is enhanced in hippocampal neurons of Fmr1-/Y mice, and presynaptic endocannabinoid -dependent LTD. It has been shown that mGluR5-coupled retrograde eCB signaling through Gq-coupled activation of PLC and diacylglycerol lipase is disrupted in both dorsal striatum and NAc, and, in contrast to hippocampus, mGluR5-dependent LTD is absent from NAc MSNs in Fmr1-/Y mice.
In dopamine biosynthesis partially improve abnormal behaviors and seizure susceptibility in Fmr1
The dopamine, glutamate, and acetylcholine systems in the brain are all affected in mice lacking Fmr1. Dopamine in particular is important for the initiation and reinforcement of motivated behaviors. Mice lacking Fmr1 have increased dopamine turnover but decreased amphetaminestimulated dopamine release in the dorsal striatum, which correlates with decreased sensitivity to amphetamine-induced motor stereotypies; as well as increased dopamine release in the prefrontal cortex. The postsynaptic effects of dopamine D1 receptor activity on AMPA-type glutamate receptor function are also reduced in both prefrontal cortex and striatum. There are relatively fewer behavioral or neurochemical studies on limbic motor system function in Fmr1-null mice than in hippocampus or neocortex. Given the critical involvement of limbic circuitry in motivation and reinforcement, changes in social integrative behavior and motor learning in FXS may be affected by underlying deficits in limbic brain reward circuitry as well as in cortex involved in memory and higher cognitive functions. Although two studies have shown normal acquisition of operant behavior using sucrose or food reinforcement in Fmr1-null mice, the neural mechanisms underlying motivation and reward have not been explored in depth in this model. Imaging studies have identified alterations in both morphology and activation patterns in the 3,4,5-Trimethoxyphenylacetic acid striatum of FXS patients, but the function of dopaminergic projections from the midbrain substantia nigra pars compacta and ventral tegmental area to their forebrain targets in the dorsal striatum and nucleus accumbens have been less extensively investigated than cortical circuits in Fmr1-null mice. Drugs that directly affect the dopamine system, including atypical neuroleptics such as aripiprazole, are of interest for the management of affective and behavioral symptoms in FXS. Cholinergic mechanisms in mesolimbic and nigrostriatal motor function, in which interactions with the dopamine system shape striatal output, are largely unexplored in this model. The goal of the current study was to characterize limbic motor circuitry with behavioral and neurochemical methods in Fmr1-null mice. Intracranial self-stimulation is an operant behavior in which animals perform a task for reinforcement by electrical brain stimulation reward. The predictable effects on BSR of drugs acting through dopamine, glutamate, or acetylcholine receptors can be compared between genotypes, and we have previously used this approach to investigate pharmacological mechanisms in other monogenic neurodevelopmental disorders. We hypothesize that Fmr1-null mice will show increased sensitivity to drugs that enhance the rewarding value of BSR and, conversely, decreased sensitivity to the reward-devaluing effects 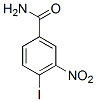 of drugs that diminish BSR. Experiments measuring the effects of the atypical neuroleptic aripiprazole, the mGluR5 antagonist MPEP, and the preferential M1 antagonist trihexyphenidyl on locomotor behavior were also performed to further differentiate drug effects on global motor function from effects specific to operant behavior. To LOUREIRIN-B determine if absence of Fmr1 alters dopaminergic neurons originating in the SNc and VTA, tyrosine hydroxylase immunoreactivity was also quantified by design-based stereology in midbrain histological sections and by western blot in tissue homogenates from dorsal striatum and NAc. We also measured the numbers of neurons expressing tyrosine hydroxylase, the rate-limiting enzyme, in the midbrain, and measured TH expression in forebrain targets of projections from those neurons. Our data suggest that absence of Fmr1 does not affect intrinsic sensitivity of mesolimbic circuits to brain stimulation reward, which we have previously shown to be increased in mice lacking the maternal allele of ubiquitin ligase, a model for Angelman syndrome.
of drugs that diminish BSR. Experiments measuring the effects of the atypical neuroleptic aripiprazole, the mGluR5 antagonist MPEP, and the preferential M1 antagonist trihexyphenidyl on locomotor behavior were also performed to further differentiate drug effects on global motor function from effects specific to operant behavior. To LOUREIRIN-B determine if absence of Fmr1 alters dopaminergic neurons originating in the SNc and VTA, tyrosine hydroxylase immunoreactivity was also quantified by design-based stereology in midbrain histological sections and by western blot in tissue homogenates from dorsal striatum and NAc. We also measured the numbers of neurons expressing tyrosine hydroxylase, the rate-limiting enzyme, in the midbrain, and measured TH expression in forebrain targets of projections from those neurons. Our data suggest that absence of Fmr1 does not affect intrinsic sensitivity of mesolimbic circuits to brain stimulation reward, which we have previously shown to be increased in mice lacking the maternal allele of ubiquitin ligase, a model for Angelman syndrome.
Pathway and network analyses generated through the use of the IPA software confirmed the major functionally related
Similar to RUNX1, the strongest  evidence for a pro-oncogenic function for RUNX2 comes from studies in lymphoma/leukemia models; however RUNX2 was also shown to play a role in invasive bone, breast, prostate, thyroid and pancreatic cancer. Lately, RUNX2 expression was also associated with EOC tumor progression and poor prognosis. This prompted us to investigate if RUNX2 is induced due to hypomethylation in advanced EOC and whether the RUNX2 gene is functionally implicated in EOC tumorigenesis, including disease progression and response to treatment. Here we show that, similar to RUNX1, the RUNX2 gene is functionally involved in EOC cell proliferation, migration and invasion. However, we also demonstrate that RUNX1 and RUNX2 employ molecular mechanisms in EOC dissemination that are specific for each gene. Snap frozen and formalin-fixed paraffin-embedded tissues of 117 serous EOC tumors were provided by the Banque de tissus et de donne��es of the Re��seau de recherche sur le cancer of the Fonds de recherche du Que��bec – Sante�� at the Hotel-Dieu de Quebec Hospital, Quebec, Canada, which is affiliated with the Canadian Tumor Repository Network. These clinical specimens included 13 borderline, or low-malignant potential tumors, 52 high-grade adenocarcinomas and 52 omental metastases. None of the patients received chemotherapy before surgery. All tumors were histologically classified according to the criteria defined by the World Health Organization. The CT treatment was completed for all patients and the response to treatment was known. Disease progression was evaluated following the guidelines of the Gynecology Cancer Intergroup. Progression free survival was defined as the time from surgery to the first observation of disease progression, recurrence or death. Thirteen normal ovarian samples and 13 normal uterine smooth muscle samples were derived from women subjected to hysterectomy with oophorectomy due to non-ovarian pathologies. TMAs were constructed, as previously described. Briefly, one representative block of each ovarian tumor and normal ovarian tissue was selected for the preparation of the tissue arrays. Three 0.6 mm cores of tumor were taken from each tumor block and placed, 0.4 mm apart, on a recipient paraffin block using a commercial tissue arrayer. The cores were randomly placed on one of two recipient blocks to avoid IHC evaluation biases. Four micron thick sections were cut for the hematoxylin-eosin staining and IHC analyses. IHC was performed, as previously described. Briefly, 4 mm tissue sections were deparaffinized and then heated in an autoclave for 12 min to retrieve the antigenicity before blocking with endogenous peroxidase. We investigated the impact of RUNX2 gene suppression on SKOV3 cell proliferation, cell cycle control, migration, invasion and sensitivity to cisplatin and paclitaxel. The RUNX2 gene knockdown led to a sharp decrease of the number of viable adherent cells, compared to control cells. This observation was further supported by the colony formation assay showing that the numbers of Albaspidin-AA clones formed by cells with stably reduced RUNX2 expression were significantly lower than that of control cells. Taken together, our observations strongly indicate an influence of RUNX2 transcripts on EOC cell proliferation and further on their Tulathromycin B propensity to form colonies. Moreover, RUNX2 suppression significantly inhibited both migration and invasion of SKOV3 cells. As shown in Figure 5A and 5B, the numbers of SKOV3 cells that passed through the filter using shRNA clones 3 and 6 were remarkably less than that in the control clone, which is indicative for a role for RUNX2 in the regulation of invasion and migration in EOC. Similar results were obtained upon RUNX2 knockdown in A2780s cells. Finally, RUNX2 suppression had no significant impact on SKOV3 cell cycle control and cisplatin and paclitaxel sensitivity.
evidence for a pro-oncogenic function for RUNX2 comes from studies in lymphoma/leukemia models; however RUNX2 was also shown to play a role in invasive bone, breast, prostate, thyroid and pancreatic cancer. Lately, RUNX2 expression was also associated with EOC tumor progression and poor prognosis. This prompted us to investigate if RUNX2 is induced due to hypomethylation in advanced EOC and whether the RUNX2 gene is functionally implicated in EOC tumorigenesis, including disease progression and response to treatment. Here we show that, similar to RUNX1, the RUNX2 gene is functionally involved in EOC cell proliferation, migration and invasion. However, we also demonstrate that RUNX1 and RUNX2 employ molecular mechanisms in EOC dissemination that are specific for each gene. Snap frozen and formalin-fixed paraffin-embedded tissues of 117 serous EOC tumors were provided by the Banque de tissus et de donne��es of the Re��seau de recherche sur le cancer of the Fonds de recherche du Que��bec – Sante�� at the Hotel-Dieu de Quebec Hospital, Quebec, Canada, which is affiliated with the Canadian Tumor Repository Network. These clinical specimens included 13 borderline, or low-malignant potential tumors, 52 high-grade adenocarcinomas and 52 omental metastases. None of the patients received chemotherapy before surgery. All tumors were histologically classified according to the criteria defined by the World Health Organization. The CT treatment was completed for all patients and the response to treatment was known. Disease progression was evaluated following the guidelines of the Gynecology Cancer Intergroup. Progression free survival was defined as the time from surgery to the first observation of disease progression, recurrence or death. Thirteen normal ovarian samples and 13 normal uterine smooth muscle samples were derived from women subjected to hysterectomy with oophorectomy due to non-ovarian pathologies. TMAs were constructed, as previously described. Briefly, one representative block of each ovarian tumor and normal ovarian tissue was selected for the preparation of the tissue arrays. Three 0.6 mm cores of tumor were taken from each tumor block and placed, 0.4 mm apart, on a recipient paraffin block using a commercial tissue arrayer. The cores were randomly placed on one of two recipient blocks to avoid IHC evaluation biases. Four micron thick sections were cut for the hematoxylin-eosin staining and IHC analyses. IHC was performed, as previously described. Briefly, 4 mm tissue sections were deparaffinized and then heated in an autoclave for 12 min to retrieve the antigenicity before blocking with endogenous peroxidase. We investigated the impact of RUNX2 gene suppression on SKOV3 cell proliferation, cell cycle control, migration, invasion and sensitivity to cisplatin and paclitaxel. The RUNX2 gene knockdown led to a sharp decrease of the number of viable adherent cells, compared to control cells. This observation was further supported by the colony formation assay showing that the numbers of Albaspidin-AA clones formed by cells with stably reduced RUNX2 expression were significantly lower than that of control cells. Taken together, our observations strongly indicate an influence of RUNX2 transcripts on EOC cell proliferation and further on their Tulathromycin B propensity to form colonies. Moreover, RUNX2 suppression significantly inhibited both migration and invasion of SKOV3 cells. As shown in Figure 5A and 5B, the numbers of SKOV3 cells that passed through the filter using shRNA clones 3 and 6 were remarkably less than that in the control clone, which is indicative for a role for RUNX2 in the regulation of invasion and migration in EOC. Similar results were obtained upon RUNX2 knockdown in A2780s cells. Finally, RUNX2 suppression had no significant impact on SKOV3 cell cycle control and cisplatin and paclitaxel sensitivity.