Palmitoylation is carried out by the DHHC-family of palmitoyltransferases. The most commonly used inhibitor of protein palmitoylation is 2bromopalmitate. However, this compound is active only at relatively high concentrations of 100 mM as a broad-spectrum inhibitor that also affects myristoylation. Other identified lipidic inhibitors were shown to exhibit only low mM activity. However, recent insight into the palmitoylation cycle of the cell has led to the development of promising inhibitors of acyl-protein thioesterase 1, which hydrolyzes the palmitoyl-ester bond. Here we report the design and application of three FRETbiosensors that can detect membrane anchorage of N-myristoylated proteins in mammalian cells. These biosensors exploit nanoclustering-induced FRET making them therefore in addition uniquely suitable for the detection of novel nanocluster modulators. Such modulators may represent a novel class of pharmacological compounds that attenuate the action of membrane anchored signaling molecules. We demonstrate that these biosensors report on the inhibition of NMTs and Met-APs and can potentially be employed in cell-based high-throughput screening. In conclusion, Yes-NANOPS is suitable for screening of chemical compound libraries and should have similar potential also for genetic screening applications. In summary, our cytometric assay merges the benefits of imaging-based high content screening and plate reader based cellular assays. The Emax value rapidly integrates essential features of the subcellular localization that is commonly obtained by cell imaging. On the other hand, the assay can be carried out at a rate comparable to that of conventional plate reader based assays. Most importantly, our assay has the unique potential for the discovery of nanoclustering modulators of myristoylated proteins, which may provide a new approach for their pharmacological Tasocitinib modulation. The importance of nanoclustering has been demonstrated for Ras signaling and by analogy, we expect that inhibition of nanoclustering of myristoylated proteins will critically affect their signaling activity, too. Our previous data showed that heterotrimeric 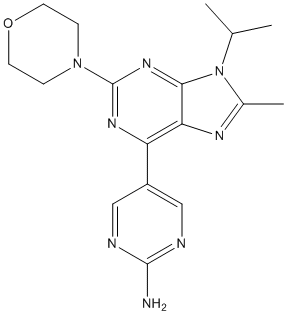 G protein alpha subunits from the Gaq and Gai/o subfamily laterally segregate into distinct membrane nanodomains. This may suggest that with the help of our FRET-biosensors inhibitors against specific nanoclusters can be developed. Our assay is flexible and can be adapted to other cell lines, provided that they allow for sufficiently high expression of the biosensor to determine the Emax parameter. It is even conceivable to implement the biosensors in protozoan pathogens, e.g. in order to understand the mechanism of action of membrane organization disrupting compounds. These features, the discovery of novel nanocluster inhibitors and the potential for a cellular high-throughput assay, clearly distinguish our assay from existing formats. The standard assay for N-myristoylation is radioactive and albeit successful even in the high-throughput setting, not really optimal towards that goal. Only recently two complementary non-radioactive in vitro assays have been published. The first detects fluorometrically the released CoA-SH and is thus generally sensitive to hydrolyzing compounds in the screening context. In the second assay a click-chemistry amenable myristate-analogue is SAR131675 utilized and detected by an ELISA-assay like procedure in both cellular and tissue samples.
G protein alpha subunits from the Gaq and Gai/o subfamily laterally segregate into distinct membrane nanodomains. This may suggest that with the help of our FRET-biosensors inhibitors against specific nanoclusters can be developed. Our assay is flexible and can be adapted to other cell lines, provided that they allow for sufficiently high expression of the biosensor to determine the Emax parameter. It is even conceivable to implement the biosensors in protozoan pathogens, e.g. in order to understand the mechanism of action of membrane organization disrupting compounds. These features, the discovery of novel nanocluster inhibitors and the potential for a cellular high-throughput assay, clearly distinguish our assay from existing formats. The standard assay for N-myristoylation is radioactive and albeit successful even in the high-throughput setting, not really optimal towards that goal. Only recently two complementary non-radioactive in vitro assays have been published. The first detects fluorometrically the released CoA-SH and is thus generally sensitive to hydrolyzing compounds in the screening context. In the second assay a click-chemistry amenable myristate-analogue is SAR131675 utilized and detected by an ELISA-assay like procedure in both cellular and tissue samples.